Current Issue
Association between Onychomycosis, Risk factors, and ABO Blood Groups
Hanan Mustafa Kalfa1, Warda Mohammed Bridan2,*
1Department of Dermatology, Faculty of Medicine, Benghazi University, Benghazi- Libya
2Department of Microbiology, Benghazi University, Benghazi- Libya
*Corresponding author: Warda Mohammed Bridan, Department of Microbiology, Benghazi University, Benghazi- Libya, Phone: 0925106588, Email: [email protected]
Received Date: September 29, 2025
Publication Date: November 07, 2025
Citation: Kalfa HM, et al. (2025). Association between Onychomycosis, Risk Factors and ABO Blood Group. Dermis. 5(6):56.
Copyright: Kalfa HM, et al. © (2025).
ABSTRACT
Background: Onychomycosis is a fungal nail infection, associated with significant morbidity and negative impact. Therefore, understanding risk factors for onychomycosis including ABO blood groups may help in prevention and management. Objective: to assess the association between onychomycosis., risk factors and ABO blood groups. Materials and Methods: A cross- sectional study conducted on 111 patients with suspected diagnosis of onychomycosis attending dermatology department Benghazi- Libya over a period of one year (2023-2024). All patients were carefully examined including detailed disease history and complete dermatological examination. Nail samples were taken for mycological tests including direct microscopic examination using potassium hydroxide 20%and culturing on Sabouraud’s dextrose agar, containing cycloheximide and chloramphenicol Blood samples were collected from all participants to determine their ABO blood groups. Results: Among the total patients, 88 (79.3%) were females and 23 (20.7) were males and their age ranged from 10 to 89 years. Direct microscopic analysis using KOH was positive in 42 (37.8%) specimens and negative in 69(62.2%) specimens. Mycological cultures were revealed growth in 111 (100%) specimens. Common risk factors in patients included tinea pedis (30.6%), nail trauma (23.5%), hyperhidrosis (17.1%), diabetes (8.1%), and psoriasis (6.3%). Other risk factors were found in (14.4%). Blood group distribution showed that blood group A was the most prevalent (40.5%), followed by group O (37%), group B (13.5%), and group AB (9%). The observed variation in the distribution of ABO blood groups was statistically significant (χ² = 34.27, df = 3, p < 0.001). Conclusions: Patients with tinea pedis, nail trauma, diabetes, and psoriasis were more prone to onychomycosis. A significant association was observed between ABO blood groups and onychomycosis mainly with blood groups A and O.
Keywords: Onychomycosis, Risk Factors, ABO Blood Groups, Tinea Pedis, Nail Trauma, Hyperhidrosis, Diabetes.
INTRODUCTION
Onychomycosis is a fungal infection of nails caused by dermatophytes, non-dermatophytes and yeasts. Onychomycosis affects approximately 5% of the population worldwide [1] and represents around 30% of all superficial fungal infection [2] and 50% of nail disorders [3]. The increased prevalence is believed to be due to some factors, including climate, geography of migration, age (increases with age), male gender, and lifestyle (sports, swimming, religious practices, and comorbidities). In particular, the following patient groups are recognized as being at greater risk for fungal infections: the elderly, patients with peripheral arterial disease, diabetes, immunodeficiency diseases, and psoriasis [4,5]. Onychomycosis is often primarily a cosmetic problem, but it is actually a debilitating disease. It has negative physical and psychological effects on the patient. Therefore, it is very important to treat fungal nail diseases [6,7].
Several risk factors have been linked to onychomycosis including tinea pedis, nail trauma, hyperhidrosis, diabetes and immunosuppressive conditions [5,8]. Identify and understanding this risk factors are essential for early diagnosis, prevention and effective management of the disease [5]. In addition to these established risk factors, the role of genetic and immunological factors including blood group antigens has been explored in various infectious diseases. The ABO blood group system is known to susceptibility to several bacterial, viral and fungal infections possibly through its impact on host immunity and pathogen adhesion mechanisms [9,10].
MATERIALS AND METHODS
Study design: A cross-sectional study was conducted including all patients with confirmed diagnosis of onychomycosis who attending dermatology department Benghazi -Libya over a period of one year (2023-2024). Each participant was carefully examined, including detailed history and complete dermatological examination.
sample size
The sample was obtained conveniently based on the frequency of all patients attending the dermatology department during the study period and was estimated to be 111 patients.
Inclusion Criteria
Patients of both sexes and different age groups who presented with clinically suspected onychomycosis and agreed to participate in the study were included.
Exclusion Criteria
Patients who had received systemic antifungal therapy within the last four weeks or topical antifungal therapy within the last one week. Patients who refused participate in the study.
Sample Collection
After obtaining verbal consent from all participants, blood samples were collected to determine their ABO blood groups. In addition, nail samples were collected directly from each patient by clipping the affected nail after cleaning the area with 70% alcohol. A new sterile nail clipper was used for each patient to prevent cross- contamination. Samples were labeled and divided into two portions: one for direct microscopic examination and the other for fungal culture.
Direct Microscopic Examination
The collected specimen was placed in a test tube and a few drops of 20% potassium hydroxide (KOH) solution were added using an eye dropper. The tube was then kept for 24 hours to dissolve the keratin. The collected specimen was mounted on glass slide and covered with a cover slip. Repeated KOH examination was performed before the specimen was considered negative for direct microscopic mount.
Cultivation of the Specimens
The specimen of each patient was placed in separate sterile Petri dish. Each specimen was inoculated on sabouraud`s dextrose Agar (SDA), and Fung biotic agar. The inoculated plates were kept in the incubator which was adjusted at 28°C and the cultures were examined every two days. The culture was considered negative if there was no growth after four weeks of incubation.
Statistical Analysis
Frequency tables and chart constructed for our data were analyzed statistically using the chi-square test. We assumed results statistically significant when P value is < 0.05. The statistical analysis of the results was carried out according to the computer package (SPSS 20.0 version).
Ethics Statement
Ethical consideration included the fact that all participants provide verbal consent to participate in the study after being informed about its purpose and procedures. The study was conducted in accordance with the ethical principle of the declaration of Helsinki. The research was authorized by the institutional Ethics committee, and confidentiality of all participants’ data was strictly maintained throughout the study. Data were used solely for scientific purposes.
RESULTS
One hundred and eleven patients with suspected onychomycosis were included in this study, including 88 (79.3%) females and 23 (20.7%) males. Their ages ranged from 10 to 89 years. Onychomycosis was most prevalent in the 30–39 age group, and female to male ratio was 3.8:1. A significant difference was observed between age and sex distribution with p value = 0.022. (Table1).
Table 1. Age and sex distribution among patients with onychomycosis
|
Age group (years) |
Male n (%) |
Female n (%) |
Total n (%) |
|
10–19 |
2 (8.7) |
1 (1.1) |
3 (2.7) |
|
20–29 |
5 (21.7) |
16 (18.2) |
21 (18.9) |
|
30–39 |
4 (17.4) |
31 (35.2) |
35 (31.5) |
|
40–49 |
3 (13.0) |
23 (26.1) |
26 (23.5) |
|
50–59 |
2 (8.7) |
10 (11.4) |
12 (10.8) |
|
60–69 |
4 (17.4) |
5 (5.7) |
9 (8.1) |
|
70–79 |
2 (8.7) |
2 (2.3) |
4 (3.6) |
|
80–89 |
1 (4.4) |
0 (0.0) |
1 (0.9) |
|
Total |
23 (100) |
88 (100) |
111 (100) |
(χ² = 16.27, df = 7, p = 0.022).
A statistically significant difference was observed between age and sex distribution.
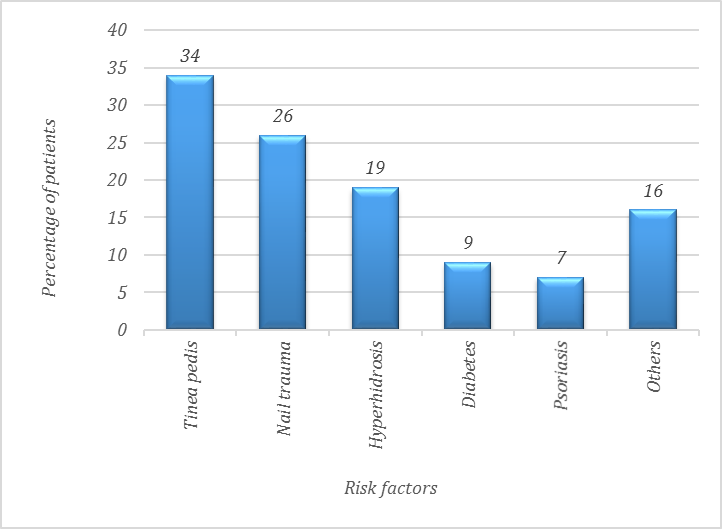
The most common risk factors were tinea pedis in 34 patients (30.6%), nail trauma in 26 (23.5%), hyperhidrosis in 19 (17.1 %), diabetes 9(8.1%) and psoriasis 7(6.3%).
Other less common factors in 16 (14.4%) cases. hand eczema in 4(3.6%), atopic dermatitis in 3 (2.7%), hypothyroidism in 2 (1.8%), rheumatoid arthritis in 2 (1.8%), pemphigus, tinea cruris, tinea corporis and tinea capitis and cancer in 1 case (0.9%) in each case (Figure 1).
There is a Chi-square test demonstrated A statistically significant difference in the distribution of risk factors indicating that some risk factors were more prevalent than others namely: Tinea pedis and nail trauma were the most common risk factors with p value < 0.001.
(χ² = 28.41, df = 5, p < 0.001).
Figure 1. Distribution of risk factors among patients with onychomycosis.
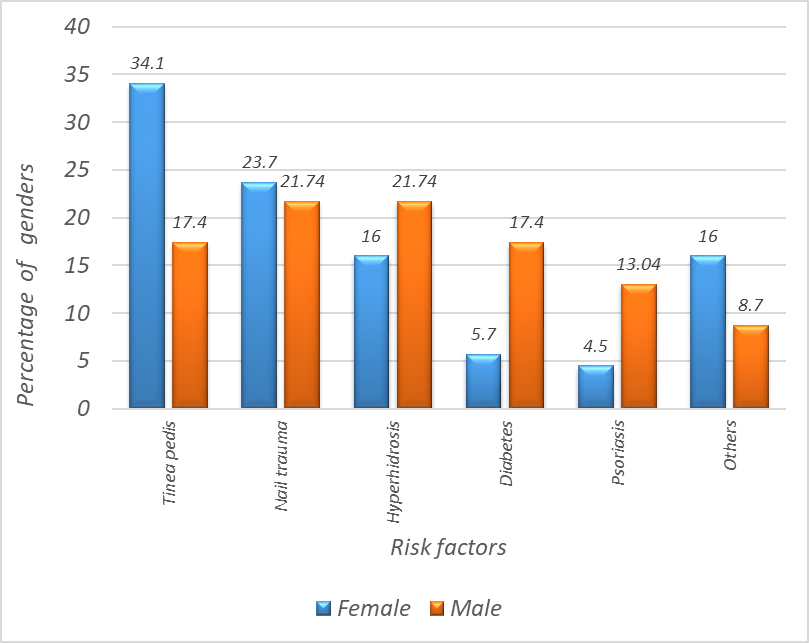
Tinea pedis was found in 34.1% of females while it was found in 17.4% of males, nail trauma was observed in 21.74% of males and in 23.7% of female's hyperhidrosis was observed in 16% of females and in 21.74% of males, Diabetes was observed in 17.4% of males and in 5.7% of females, psoriasis was found in 13.04% of males and 4.5% of females. Other factors showed no significant association with sex p value = 0.163) (Figure 2).
(χ² = 7.89, df = 5, p = 0.163).
Figure 2. Risk factors and sex distribution among patients with onychomycosis.
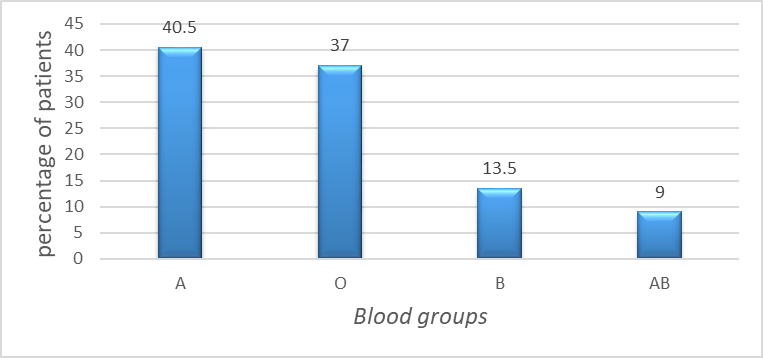
Among the blood types, type A was observed in 45 patients (40.5%), type O in 41(37 %), type B in 15 (13.5%) and type AB which was the least common in 10 (9%) (Figure 3).
Therefore, blood groups A and O were significantly more frequent, while AB was the least among the study population with highly statistical significant association (p < 0.001) between ABO blood groups and onychomycosis.
The percentages obtained are based on the study participants only and may not reflect the general population. This finding suggests that individuals with blood groups A and O may have a higher predisposition to onychomycosis compared to those with groups B and AB.
(χ² = 34.27, df = 3, p < 0.001).
Figure 3. Distribution of ABO blood groups among patients with onychomycosis.
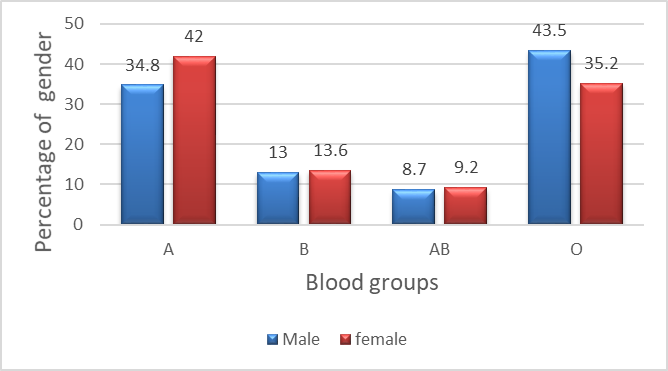
Onychomycosis in blood type A found in 42% of females and in 34.8% of males, onychomycosis blood type O was observed in 43.5% of males and in 35.2% in females. Blood type B was found in13.6% of females and in 13% of males, and blood type AB in 9.2% of females and 8.7% of males (Figure 4). There is no statistically significant difference between males and females in the distribution of blood groups (p > 0.05).
(χ² = 0.58, df = 3, p = 0.901).
Figure 4. Distribution of ABO blood groups according to sex among patients with onychomycosis.
In current study, which study found no statistically significant association between risk factors and ABO blood groups in females (χ² = 18.24, df = 15, p = 0.250). While none of the individual risk factors demonstrated a significant relationship, Tinea pedis (p = 0.078) and others (p = 0.060) were close to the threshold of 0.05. These borderline results may suggest a possible trend toward an association; however, they should be interpreted with caution, as they do not meet the criteria for statistical significance and may require larger sample sizes or further studies to be confirmed. Exhibited borderline values (Table 2).
Table 2. Distribution of risk factors according to ABO blood groups among females with onychomycosis (n = 88)
|
Risk factors |
A n (%) |
B n (%) |
AB n (%) |
O n (%) |
Total n (%) |
p-value |
|---|---|---|---|---|---|---|
|
Tinea pedis |
8 (26.6) |
6 (20.0) |
5 (16.7) |
11 (36.7) |
30 (34.1) |
0.078 |
|
Nail trauma |
8 (38.1) |
4 (19.1) |
2 (9.5) |
7 (33.3) |
21 (23.9) |
0.867 |
|
Hyperhidrosis |
7 (50.0) |
0 (0.0) |
1 (7.0) |
6 (43.0) |
14 (15.9) |
0.413 |
|
Diabetes |
2 (40.0) |
2 (40.0) |
0 (0.0) |
1 (20.0) |
5 (5.7) |
0.316 |
|
Psoriasis |
2 (50.0) |
0 (0.0) |
0 (0.0) |
2 (50.0) |
4 (4.5) |
0.735 |
|
Others |
10 (71.4) |
0 (0.0) |
0 (0.0) |
4 (28.6) |
14 (15.9) |
0.060 |
|
Total |
37 (42.0) |
12 (13.6) |
8 (9.1) |
31 (35.2) |
88 (100) |
— |
(χ² = 18.24, df = 15, p = 0.250) No statistically significant association was found.
Also, Chi-square analysis showed no statistically significant association between risk factors and blood groups in males (Chi-square = 7.82, df = 15, p = 0.931). All p-values were greater than 0.05, indicating that there were no statistically significant differences in the distribution of risk factors among the different blood groups (Table 3).
Table 3. Distribution of risk factors according to ABO blood groups among male patients with onychomycosis (n = 23)
|
Risk factors |
A (%) |
B (%) |
AB (%) |
O (%) |
Total (%) |
p-value |
|
Tinea pedis |
2 (50%) |
1 (25%) |
0 (0%) |
1 (25%) |
4 (17.4%) |
0.421 |
|
Nail trauma |
2 (40%) |
0 (0%) |
1 (20%) |
2 (40%) |
5 (21.7%) |
0.835 |
|
Hyperhidrosis |
1 (20%) |
1 (20%) |
0 (0%) |
3 (60%) |
5 (21.7%) |
0.661 |
|
Diabetes |
1 (25%) |
0 (0%) |
1 (25%) |
2 (50%) |
4 (17.4%) |
0.769 |
|
Psoriasis |
1 (33.3%) |
1 (33.3%) |
0 (0%) |
1 (33.3%) |
3 (13.0%) |
0.758 |
|
Others |
1 (50%) |
0 (0%) |
0 (0%) |
1 (50%) |
2 (8.7%) |
0.886 |
|
Total |
4 (100%) |
5 (100%) |
5 (100%) |
4 (100%) |
23 (100%) |
— |
(χ² = 7.82, df = 15, p = 0.931). No statistically significant association
DISCUSSION
Onychomycosis is a chronic infection of the nails, and is considered a serious problem for public health [8]. Onychomycosis has both psychosocial and physical detrimental effects on quality of life [11]. In this study, we studied the association between risk factors and blood types with onychomycosis. Among the factors associated with onychomycosis in younger individuals (31.5%) in our study, similar findings also reported in studies from Saudi Arabia and Indonesia [12,13]. In contrast, some studies have reported that onychomycosis affected elderly individuals [14]. The younger age groups observed in current study may be considered more susceptible to occupational exposure associated with trauma or more common used of occlusive foot wear.
Tinea pedis is a superficial fungal infection of the nails [15]. Tinea pedis, or athlete’s foot, is the most common caused by dermatophytes infection. It tends to occur most often in men aged 20–40 years and usually relates to sweating and warmth [15,16]. The Patients with a history of fungal infections on the feet were at greater risk of developing onychomycosis. In the present study, we found that tinea pedis (30.6%) was closely associated with onychomycosis. Similarly, in a prospective survey of 2,761 patients, 42.8% had both onychomycosis and tinea pedis [17]. Although tinea pedis is generally more common in males, our study revealed that it was more frequent in women (34.1%) compared to men (17.4%). Frequent manicure and pedicure in women may increase the risk of fungal nail infections by damaging the cuticle. Also moist environment, tight shoes, and frequent exposure to detergents increase the risk of fungal nail infection.
In the current study, we observed other risk factors in 16 cases including hand eczema in 4 cases (3.6%), atopic dermatitis in 3 case (2.7%), hypothyroidism in 2 cases (1.8%), and rheumatoid arthritis in 2 case (1.8%). Pemphigus, tinea cruris, tinea corporis and tinea capitis and cancer each had 1 case (.9%), with a low association with disease [18]. Trauma to the nails can be an entry point for fungi, increasing the risk of a fungal infection. In the present study, we found that nail trauma (23.4%) was associated with onychomycosis. The exact mechanism of trauma that might make this nail more susceptible to fungal infection is not known. It can be hypothesized that neurovascular changes after trauma may lead to impaired blood supply and decreased immune response to fungal infection in the affected area [19].
Hyperhidrosis is a disorder of excessive sweating due to overstimulation of cholinergic receptors on the sweat glands. In our study; it was observed in (17.2%) of the cases. This disorder is characterized by sweating beyond what the body uses for homeostatic temperature regulation [20]. Eccrine glands are concentrated in areas such as the axillae, palms, soles, and face, which are most commonly associated with hyperhidrosis [21]. Diabetes is a risk factor that increases the likelihood of developing onychomycosis. Diabetic’s patients present with several clinical conditions that contribute to the development of onychomycosis. Elevated hemoglobin (HbA1C) levels and prolonged diabetes lead to thickened nails and accelerated subungual keratinization. These conditions increase the likelihood of developing onychomycosis [22,23].
In multicenter study, diabetic patients were found to be approximately 2.8 times more likely to develop toenail onychomycosis compared to non-diabetic individuals [24]. In current the study, we found that diabetes mellitus (8.1%) was associated with onychomycosis, which was reported in some studies as 9.2% and in others as 22% [14,25]. Onychomycosis is more common in patients with psoriasis than in the rest of the population, with an estimated prevalence ranging between 13.1% and 22% [26,27]. In our study, onychomycosis was detected in (6.3%) of psoriatic patients, indicated that psoriasis may represent one of the risk factors associated with onychomycosis. However, despite the heterogeneity of studies, the results of which have sometimes been controversial, there is a strong indication that there may be an association between psoriasis and onychomycosis, as well as the frequency variations observed between geographical areas [28].
Some studies have explored the association between AB0 blood types and susceptibility to infection, but the results have been inconsistent [9]. Baqir et al. [9] reported that women with blood types O were more likely to develop urinary tract infection. Other studies have shown that having blood type A can be a risk factor for Candida albicans infection [29]. Some studies have observed antigenic similarities between human erythrocyte antigens A1 and A2 and cell wall glycoproteins of Trichophyton species. Young and Roth [10] first reported in 1979 that a glycoprotein isolated from the cell wall of T. mentagrophytes cross-reacted with human blood group isoantigen A1 and A2, suggestive potential immunological cross- reactivity. Furthermore, individuals with blood type A more susceptible to chronic skin infections which may be related to this immunological reactivity [10]. While Nehring [30] found no evidence of increased susceptibility of individuals with blood type A to dermatophyte infection, more recent studies have explored the association between ABO blood types and susceptibility to dermatophytes infection. Ahmed et al. [31] reported that certain blood groups were significantly associated with specific types of dermatophytosis, supporting the idea that blood group antigens may influence host susceptibility. The results obtained in our study indicate that Although blood group A was the most frequent among patients with onychomycosis (40.5%), the analysis revealed a significant association between ABO blood groups and susceptibility to the disease.
In current study, females with blood type A and males with type blood type O were more likely to develop onychomycosis. Similarly, another study found that male patients with blood groups O and females with blood group A had the highest incidence of the disease [31].
CONCLUSIONS
Tinea pedis and nail trauma were the most common risk factors for onychomycosis. A significant difference was observed in the distribution of ABO blood groups with patients of blood groups A and O being more frequently affected by onychomycosis.
LIMITATIONS
This study has several limitations. First, the sample size was relatively small and limited to a single center, which may reduce the generalizability of the findings. Second, only patients attending the dermatology department were included, which may not represent the general population. Third, the cross-sectional design did not allow for establishing causal relationships between risk factors and onychomycosis. Finally, some potential confounding variables, such as occupation, footwear habits, and hygiene practices, were not fully assessed. Future multicenter studies with larger sample sizes and prospective designs are recommended to confirm these findings.
ACKNOWLEDGEMENTS
None.
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest.
REFERENCES
- Leung AKC, Lam JM. Leong KF, Hon KL, Barankin B, Leung AA. (2020). Onychomycosis: an updated review. Recent Pat Inflamm Allergy Drug Discov. 14 (1):32-45.
- Singal A, Khanna D. (2011). Onychomycosis: Diagnosis and Management. Indian J Dermatol Venereol Leprol. 77:659-672.
- Vasava D, Mehta H, Patel T, Jhavar M, Lakhotia R. (2021). Clinical Thermoscopic, and Mycological Association in Onychomycosis in Tertiary care hospital. Clin Dermatol Rev. 5(1):43-98.
- Lipner SR, Scher. RK. (2023). Onychomycosis: Epidemiology, Diagnosis, and treatment in changing landscape. J Drugs Dermatol. 22(3):223-229.
- Pappas PG, Kaur R, Gupta AK. (2022). Fungal infections and Nail Psoriasis: An update. J Fungi (Basal). 8(2):154.
- Debbagh F, Babokh F, Sbai M, El Mezouari M, Moutaj R. (2023). Impact of onychomycosis on the quality of life of patients. Curr Med Mycol. (2):39-44.
- Stewart C, Algu L, Kamran R, Lipner S. (2020). Impact of Onychomycosis and treatment on patient-reported quality of life outcomes: A systematic review. J Acad Dermatol. 82(2):438-448.
- Albucker SJ, Smith T, Johnson R, et al. (2023). Risk factors and treatment trends for Onychomycosis. J Clin Med. 12(4):1234.
- Baqir QK, Baqer GK, Baqer FK, Abbas BA. (2024). Relationship of ABO and Lewis blood groups in patients with urinary tract infection. Iran J War Public Health. 16(1):43-48.
- Young E, Roth FJ. (1979). Immunological cross-reactivity between a glycoprotein isolated from Trichophyton mentagrophytes and human isoantigen A. J invest Dermatol. 72:46-51.
- Gupta AK, Mays RR. (2018). The Impact of Onychomycosis on Quality of Life: A Systematic Review of the Available Literature. Skin Appendage Disord. 4(4):208-216.
- Al-Sheikh H. (2009). Epidemiology of dermatophytes in Eastern Province of Saudi Arabia. Res J Microbiol. 4(6):229-234.
- Bramono K, Budimuja U. (2005). Epidemiology of onychomycosis in Indonesia: data obtained from three individual studies. Jpn J Med Mycol. 46(3):171-176.
- EL Fekih N, Belghith I, Trabelsi S, Skhiri-Aounallah H, Khaled S, Fazaa B. (2012). Epidemiological and etiological study of foot mycosis in Tunisia. Actas Dermosifiliogr. 103:520-524.
- Lipner SR, Scher RK. (2015). Management of onychomycosis and co-existing tinea pedis. J Drugs Dermatol. 14(5):492-494.
- SahooAK, Mahajan R. (2024). Tinea pedis: an update review. J Fungi (Basel). 10(2):149.
- Szepietowski JC, Reich A, Garlowska E, Kulig M, Baran E; Onychomycosis Epidemiology Study Group. (2006). Factors influencing Coexistence of Toenail Onychomycosis With Tinea Pedis and Other Dermatomycoses: A Survey of 2761 Patients. Arch Dermatol. 142(10):1279-1284.
- Haneke E, Stovbyr G. (2023). Post-Traumatic Single-Digit Onychomycosis. J Fungi (Basel). 9(3):313.
- Albucker SJ, Falotico JM, Choo ZN, Matushansky JT, Lipner SR. (2023). Risk Factors and Treatment Trends for Onychomycosis: A Case-Control Study of Onychomycosis Patients in the All of Us Research Program. J Fungi (Basel). 9(7):712.
- Sammons JE, Khachemoune A. (2017). Axillary hyperhidrosis: a focused review. J Dermatolog Treat. 28(7):582-590.
- Menzinger S, Quenan S. (2017). Evaluation and management of hyperhidrosis. Rev Med Suisse. 13(556):710-714.
- Trovato L, Calvo M, DE Pasquale R, Scalia G, Oliveri S. (2022). Prevalence of onychomycosis in diabetic patients: a case control study performed at university hospital policlinico in Catania. J Fungi(Basel). 8(9):922.
- Rodrigues CF, Rodrigues ME, Henriques M. (2019). Candida sp. Infections in Patients with Diabetes Mellitus. J Clin Med. 8:76.
- Bersano JM, de Almeida JR, de Brito JMC, Lemos AC, Galdino ACM, Silva D. (2023). Prevalence and risk predictors of onychomycosis in patients with type 2 diabetes mellitus: a multicenter study. Front Med (Lausanne). 10:1268324.
- Mahwash R, Faria A, Bushra B, Zahida R. (2017). Frequency factors of onychomycosis. J Pak Assoc Dermatol. 27(3):226-231.
- Kaçar N, Ergin S, Ergin C, Erdogan BS, Kaleli I. (2007). The prevalence, aetiological agents and therapy of onychomycosis in patients with psoriasis: a prospective controlled trial. Clin Exp Dermatol. 32(1):1-5.
- Abdo HM, Hassab-El-Naby HM, Bashtar MR, Hasan MS, Elsaie ML. (2024). Prevalence of onychomycosis among psoriasis patients: a clinico-mycological and dermoscopic comparative cross sectional study. Sci Rep. 14(1):21743.
- Klaassen KM, Dulak MG, van de Kerkhof PC, Pasch MC. (2014). The prevalence of onychomycosis in psoriatic patients: a systematic review. J Eur Acad Dermatol Venereol. 28(5):533-541.
- Thanomsridetcha N, Tanakittyakul P, Sub-In P, Pichaipaet P, Tangwattanachuleeporn M, Kitisin T, et al. (2023). The Potential Association of Human ABO Blood Group in Candida albicans Germination. Trends sci. 20(12):7116.
- Nehring N. (1979). Chronic Trichophyton rubrum infections and blood groups. J invest Dermatol. 73(3):392-394.
- Ahmed MNU, Yousaf M, Gomes RR, Rahman K, Mahmud H, Johora FT, et al. (2014). Association between Blood Group Typing and clinical manifestation of Dermatophytosis patients. JAFMC Bangladesh. 20(2):22-26.
 Abstract
Abstract  PDF
PDF